Measuring Skeletal Muscle Oxidative Capacity with NIRS
Near-infrared spectroscopy (NIRS) is a powerful non-invasive tool that allows researchers to measure, in real-time, the changes in oxygen dynamics in a myriad of tissues during a variety of interventions from sitting to exercising. One of them most useful functions of NIRS is its utility in measuring skeletal muscle oxidative capacity. This technique has even been validated against phosphorus magnetic resonance spectroscopy (P-MRS) and high resolution respirometry (HRR) which are considered the gold-standard measurements for mitochondrial function. In both of the above studies the NIRS technique, described in detail below, was shown to be significantly correlated to each gold-standard measure (r > .88, p < 0.0001) and (r = 0.61 – 0.74, p < 0.01), respectively. Very briefly, to measure skeletal muscle oxidative capacity, a seated participant contracts their muscle (to provide a metabolic stimulus), then undergoes a series of 5-15 rapid cuff inflations, from a blood pressure cuff located proximal to the area of interrogation. Upon completion the cuff/s are inflated above systolic blood pressure for 3-5 minutes in order to normalize the NIRS signal. Finally, the slope of each cuff is measured, plotted, and fit to a monoexponential curve allowing for the determination of the NIRS rate constant, k, which is the value used as a surrogate for skeletal muscle oxidative capacity.
List of references for more in-depth methodological descriptions:
- Ryan et al. 2012 – Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes
- Ryan et al. 2014 - Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements – Probably the best description of the methods
- Ryan et al. 2013 - A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy
- Brizendine et al. 2013 - Skeletal Muscle Metabolism in Endurance Athletes with Near-Infrared Spectroscopy
- McCully and Ryan Patent - Measuring Mitochondrial Capacity
Materials. To measure skeletal muscle oxidative capacity you will need the following:
- Rapid-inflation blood pressure cuff system (Hokanson is typically used for this).
- NIRS device (most continuous-wave devices require the calibration step below).
- Chair or apparatus to keep the leg or arm secure and in place during the cuffs.
- Skin Calipers or Ultrasound Machine to measure adipose tissue thickness.
Methods – In the Lab.
Measuring Adipose Tissue Thickness. Adipose tissue thickness (ATT) should be measured as close to the site of the NIRS device as possible. Measuring ATT allows for some control over the depth to which the NIRS signal is penetrating into the skeletal muscle. It also will give researchers confidence that the majority of the signal is coming from muscle. For example, when using an adjustable NIRS device, the first detector spacing is set to (ATT + 5mm)*2 then the second spacing is set to ((ATT + 5mm)*2) + 1cm this will ensure most of the signal is making it into the muscle.
Arterial Occlusions. Arterial occlusions require a blood pressure cuff to be inflated to above systolic blood pressure, as to totally stop the flow of blood into and out of the tissue of interest. Many publications recommend using a rapid inflation cuff, placed proximal to the tissue of interest, that can be inflated to 300mmHg. The arterial occlusion protocol is as follows:
- After a 15s isometric contraction or electrical stimulation of the muscle (it doesn’t matter which - Ryan et al. 2013) apply multiple (5-20) arterial occlusions as follows:
- Seat the participant in a chair, with their ankle immobilized, so they can relax fully and minimize movement artifacts.
- Complete 2 x 30s Resting Cuffs (resting mVO2 is an important normalization step for analysis).
- Complete a brief 15-20s isometric contraction or electrical stimulation on the muscle of interest (it doesn’t matter which - Ryan et al. 2013) in order to increase oxygen consumption.
- Complete a series of arterial occlusions (300mmHg) to get (muscular rate of oxygen consumption) mO2:
- 5 x 5s on/off
- 5 x 10s on/off
- 5 x 15s on/off
- Repeat step iv
Ischemic Calibration. Continuous wave NIRS devices are unable to measure exact concentrations of oxy and deoxy hemoglobin and myoglobin, so the typical data output is in measured as changes in the NIRS signal. Therefore, an ischemic calibration is necessary to be able to compare NIRS signals between participants and between trials. An ischemic calibration involves inflating the pressure cuffs to 300mmHg for 3-5 minutes. During the 3-5 minutes a nadir in NIRS signal will occur, this is assumed to be the 0% oxygenation mark. Upon release of the cuff a hyperemic response will occur producing a zenith in the NIRS signal, which is then used as the 100% oxygenation point for normalization. Complete the ischemic calibration as follows:
- Immediately following the second series of arterial occlusions, inflate the cuffs to 300mmHg for 3-5 minutes.
- Once the signal flattens out and stops changing release the cuff.
- Keep the participant sitting until the signal reaches a peak and begins to come back down.
Data Analysis.
- After data is gathered:
- If CW-NIRS is used: Use ischemic calibration minimum point assumed to be 0% oxygenation, maximum point assumed to be 100% oxygenation to get NIRS signal as percentage.
- If Frequency Domain or Moxy is Used: No calibration changes needed
- Correct for blood-volume changes as per (a) and (e), above. Note: this is a patented process.
- Analyze each cuff for slope (aka mO2 calculation) of first 3s of each cuff.
- Graph all mVO2 values for 15 cuffs. Fit a monoexponential function to the graph. The function should look something like:
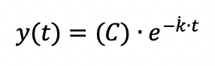
C represents a constant, determined by the monoexponential fit, is the fitting rate constant (or time constant) for the recovery of mO2, and t is the time.
Summary. Measuring skeletal muscle oxidative capacity no longer requires a participant to be isolated in a magnetic resonance spectroscopy machine, or to give a skeletal muscle biopsy. While these methods are still held as the gold standard for measuring skeletal muscle mitochondria oxygen capacity and kinetics, NIRS devices provide adequate utility to evaluate skeletal muscle function as it pertains to oxygen delivery and utilization. As NIRS devices become more portable and accurate this evaluation will only become more important as skeletal muscle function/capacity is a huge indicator of health.